Chemical Treatment Methods

Safe drinking water and effective wastewater treatment are critical for protecting public health and the environment. Chemical technologies play an important role alongside physical and biological treatment methods. This article provides an overview of key chemical processes used, design considerations, and challenges for further improvement.
Chemically-enhanced sedimentation (coagulation and flocculation) is used to remove suspended solids and enhance subsequent treatment steps. Chemicals such as lime, alum, and cationic/anionic polymers destabilize colloidal particles (0.01-1 μm) to form settleable flocs. Up to 90% removal of total suspended solids (60-90%) and phosphorus (70-90%) can be achieved.
Chemical precipitation with lime or metal salts like ferric chloride targets phosphorus, metals like zinc and copper, and other contaminants. It is a key step for controlling nutrient discharges to below 1 mg/L total phosphorus.
Disinfection uses oxidizing agents like chlorine (100-200 mg/L), ozone (0.5-5 mg/L), or UV radiation (100-5000 J/m2) to inactivate pathogens. A chlorine residual of 0.2-0.5 mg/L helps prevent regrowth. Concerns over disinfection byproducts have increased interest in ozone and UV approaches. Advanced oxidation processes (AOPs) using ozone, UV, or catalysts to generate hydroxyl radicals can also transform recalcitrant organic compounds.
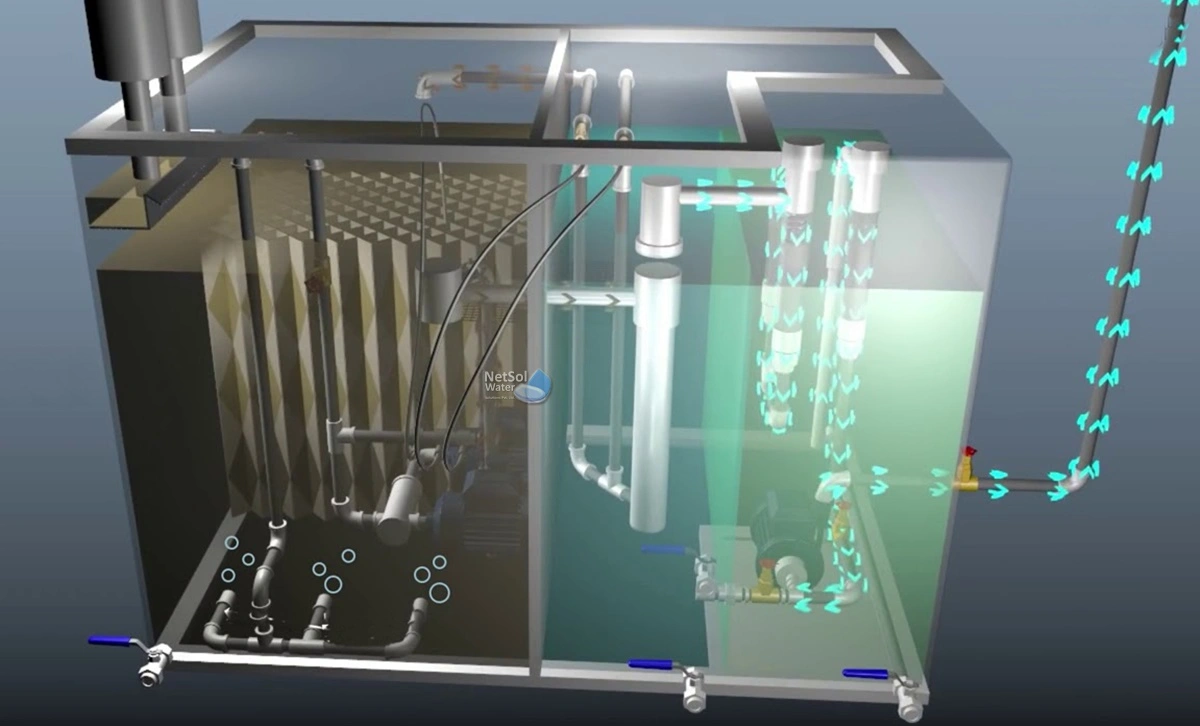
Design and Operational Considerations
Key factors involve determining influent volumes/variability, analyzing waste production, ensuring reliability through redundancy, and evaluating environmental impacts. Understanding influent characteristics is vital for process selection and sizing. Jar testing helps optimize 20-200 mg/L chemical doses. Monitoring all process streams is necessary.
Challenges and Future Trends
Removing trace emerging contaminants (< 1 μg/L) and minimizing residual toxicity are becoming priorities, but the vast number of chemicals (>100,000 commercialized) poses prioritization and risk assessment challenges. Implementing advanced treatment globally also faces difficulties. However, water reuse and multi-barrier concepts are gaining support. Balancing treatment effectiveness and sustainability will shape further advances.
Conclusion
Chemical methods present indispensable treatment mechanisms but also implementation and contaminant challenges. Advances for removing priority pollutants while supporting water conservation and reuse efforts will require integrative social and technological solutions. Ongoing research across chemical, physical, and biological processes will be vital for affordable innovations that protect ecosystem and public health.
